On Earth, from microorganisms in the soil to human organs, a variety of cells, having some relationships with each other, work to maintain homeostasis. In our laboratory, to study various cellular functions in nature or human body, "single cellular engineering" and "complex cellular engineering" are integrated based on systems theory: the former picks up a single cell and checks out its structure or function; and the latter investigates the functional relationships between individual cells in microbial communities or tissues. The target cells are microbial cells and animal cells. Also, we conducted research on cross-talk between the two. As shown above, we focus on the various cellular functions. Then, we are conducting multidisciplinary researches of various stages, such as development of methodology for cell functional analysis, elucidation of molecular mechanisms in the cells, and development of applied technology for medical care, health care, food industry and environment conservation.
Cellular Biotechnology Laboratory
Research Groups
1, Gut homeostasis through host-microbes interactions.
Commensal bacteria colonized symbiotically in our body are closely related to the human health and disease. The mucosal epithelium is continuously exposed to the antigen- and microbe-rich environment. Nevertheless, numerous commensal bacteria do not elicit the immune inflammation. Thus, it is hypothesized that the mucosal epithelial cells may equip with the mechanism to prevent or limit activation of immune inflammation and fortify the epithelial barrier function through host-microbe interactions, and this interaction plays role in the maintenance of intestinal epithelial homeostasis. For the prophylaxis and treatment of epithelial infection by pathogenic bacteria, the therapeutic strategy can be divided into two different strategies. One is to selectively eliminate of infectious bacteria with antibiotics and vaccine. Other is to control the immune function, i.e. the suppression of proinflammatory mediator production and the fortify of the epithelial barrier and innate immune response. In the chronic inflammatory diseases which have no specific treatment, the latter strategy is important to improve patient’s quality of life. Now, we take up the investigation to unlock the mechanism to maintain the gut homeostasis through host-microbe interactions.
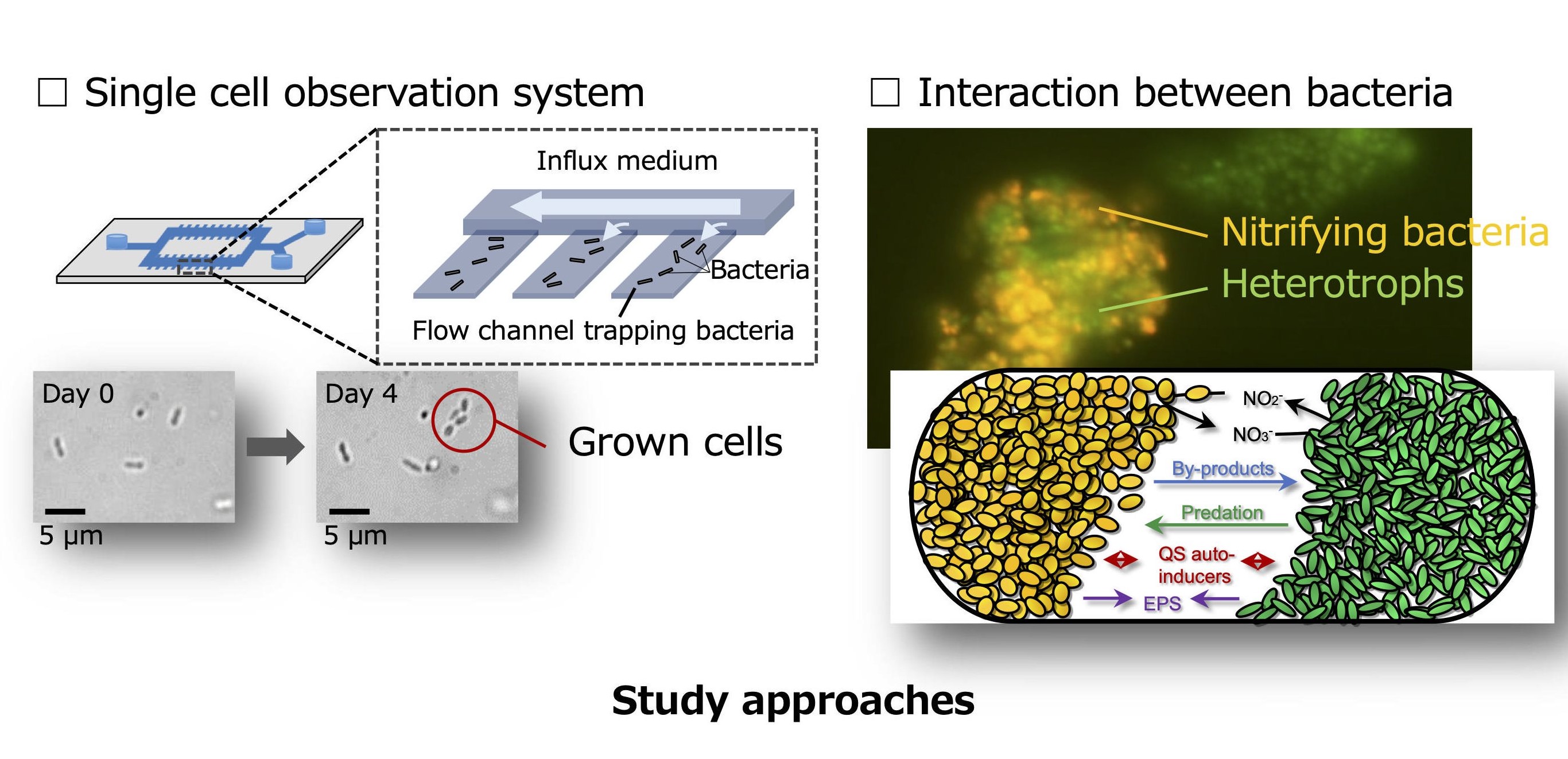
2, Development of novel isolation methods for uncultured microbes and physiological and genomic characteristics of novel nitrifiers.
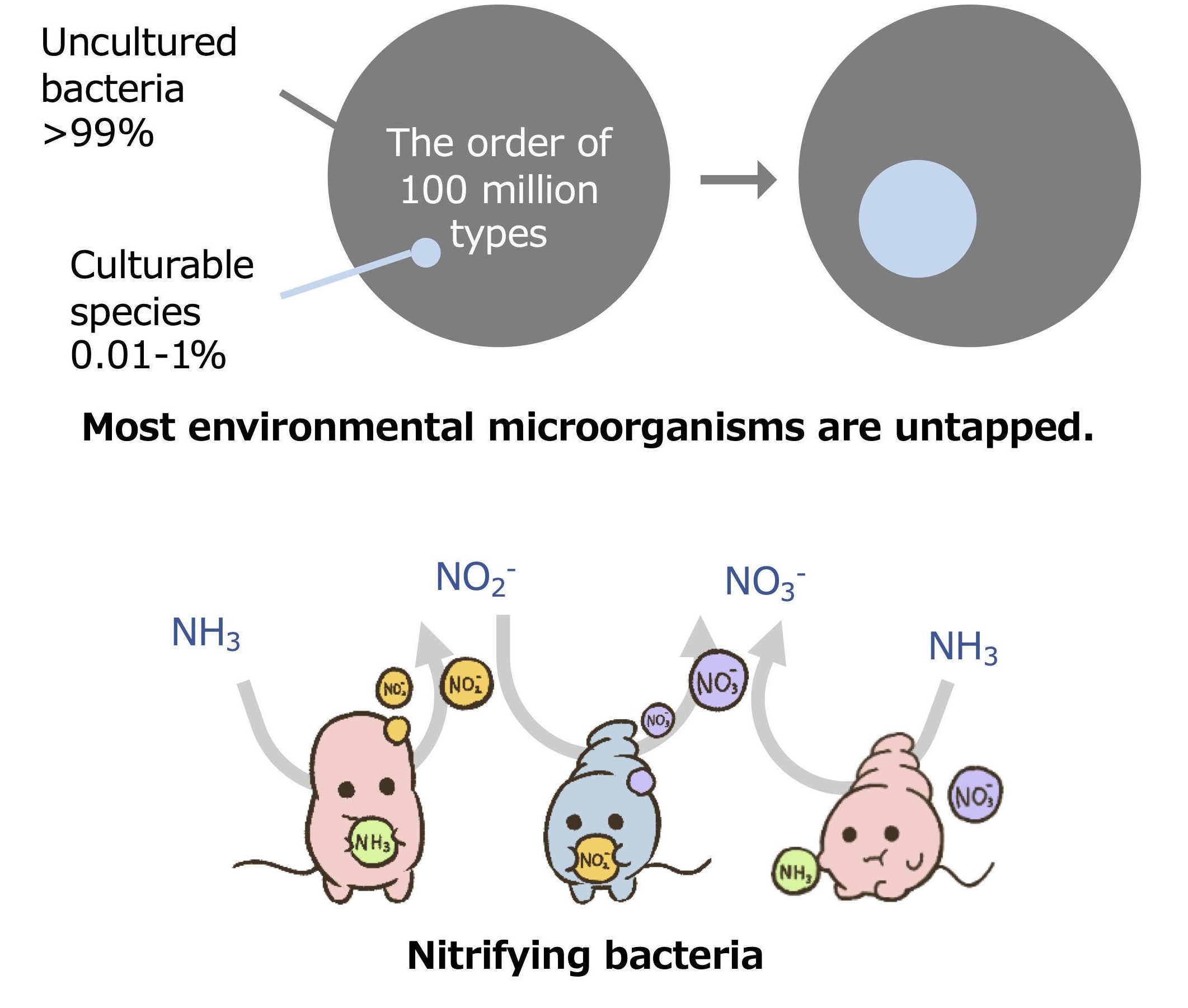
Majority of microorganisms found in environments cannot grow under laboratory conditions. Although only a fewspecies can be cultivated in artificial media, environmental microorganisms have benefited a wide range of fields,including food, medicine, and environmental engineering. Our mission is to contribute to the exploitation ofuntapped bioresources by increasing the number of culturable microbial species.
Recently, next-generation sequencers enable to access genomic information that is the blueprint of themicroorganisms and to predict their microbial ecology without cultivation. However, it is necessary to cultivatemicroorganisms to obtain the key information on physiological activity and functional metabolic pathways ofindividual microorganisms, which are essential for the industrial use. Cultivation-dependent studies using isolatedpure cultures will provide insight into the physiological characteristics of the microorganisms.
Our group has focused on environmentally important nitrifying bacteria as a model for “fastidious”microorganisms. We have developed innovative cultivation strategies and isolated nitrifying bacterial strains fromactivated sludge, soil, and marine. Identification of the reasons for the difficulty in cultivating the nitrifying bacteriacan lead to estimate the factors that control the growth of other fastidious environmental microorganisms.
Our goal is to find the answer to the question: why are most environmental microorganisms difficult to cultivate?We are now investigating growth heterogeneity and microbial interactions of the nitrifying bacteria and elucidatingthe causes of the difficulty in cultivating environmental microorganisms.
3, Development of biofilm
Approximately 90 % of all bacterial species has been believed to live as a form of community, “Biofilm”, that can adsorb on the surface of the boundary between solid and liquid phases and is surrounded by various gel-like organic compounds derived from biomass. Therefore, biofilm can exist everywhere such as sea, lake, and within human body.
I. Spatio-temporal dynamics of dormant cells within biofilmsBiofilm formation is a kind of bacterial survival strategy. As seen in other species, bacterial cells that live as a biofilm community is known to be tolerant against various environmental stresses rather than living as a “single and lonely” form. We now have been focusing on dormant and antibiotic-tolerant cells in the biofilm as one of the causes of antibiotic failure. Many researchers reported that most cells within biofilm can’t grow and devide because of the environmental stresses such as low-nutrient, anaerobic, and highly-densed conditions. These dormant cells are inactive to any antibiotics targeting bacterial growth system. In our research, we aim to develop a growth marker that can visualize when, where, and how dormant cells are formed in biofilm community and survive antibiotics. To tackle this issue, we have been working on researchby combining with genetical modification and mathematical simulation technique.
II. Molecular mechanism underlying dormant antibiotic-tolerant cellsWe now are interested in the question, “Why can bacteria form dormant cells?”. Dormant cells can emerge not only within biofilm but also in a clonal population in butch culture, called persister. Dormant persisters are phenotypic variants without any mutation, so they are different from resistant cells. Although recent studies have shown that persister formation can be induced by multiple pathways, the general understanding of mechanism underlying persister formation is still elusive.In recent years, we have a methodological problem about distinguishing dormant cells and actively growing cells so far, because of its poor knowledge about persister. In our research, we have developed a novel approach that can visualize and identify dormant cells, and we are analyzing the molecular mechanism using this method.
- TOP
- Copyright © Tsuneda Lab. Waseda Univ. All rights reserved.

